Copyright © 2022 Binhui Bio All Rights Reserved
鄂ICP备16007335号-1Legal Terms
Partners
Four
PlatformsThrough the accumulation of technology, talents, and management experience gained over the last ten years in the R&D and industrialization of innovative anti-tumor drugs, Binhui Bio has developed a mature technical service platform for viral vectors, nucleic acid drugs, protein drugs, and cell therapies.

Viral Vector Platform

Nucleic Acid Platform


Protein Platform

Cell Therapy Platform
Binhui's Viral Vector Platform
The highly scalable oHSV2-based platform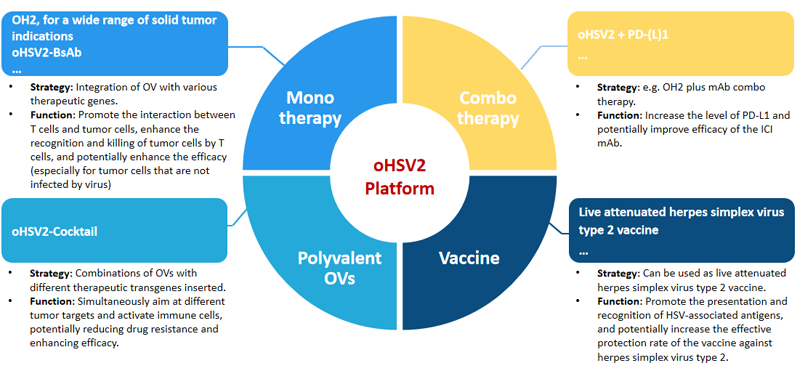

1)Deletion of neurotoxic factor ICP34.5, promote selective viral replication in tumor cells, enhance safety.
2)Deletion of immune suppressive gene ICP47, promote antigen presentation and viral replication, enhance anti-tumor efficacy.
3)Insert GM-CSF, to induce stronger, durable and specific anti-tumor immune responses.
Viral Vector Platform Progress
Candidates
Indication
Pre-clinical
IND
Dose Escalation (Phase I)
Dose Extension (Phase II)
Pivotal Study (Phase III)
Melanoma
GBM
mCRC
Others
Melanoma with αPD-1 failure
mCRC
Sarcoma
Solid tumors(IIT)
Solid tumors
Solid tumors
BH-RACE platform of nucleic acid drug
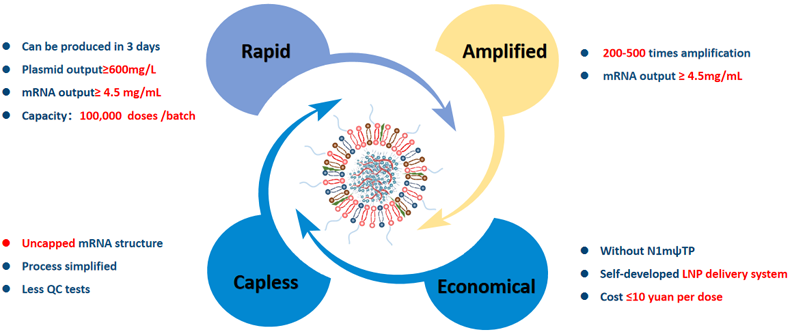
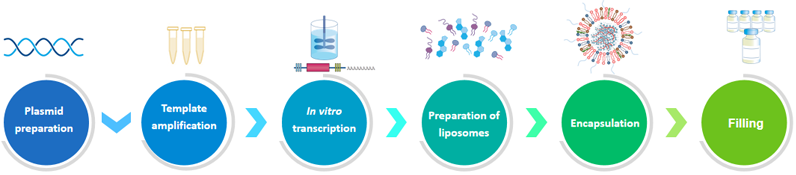
Product process
S-protein trimer mRNA vaccine
Monkeypox vaccine
Rabies vaccine
Neo-antigen tumor vaccines
Heterologous tumor mRNA vaccines
In vivo CAR-T
In vivo CAR-NK
Nucleic Acid Platform Progress
Candidates
Indication
Pre-clinical
IND
Dose Escalation (Phase I)
Dose Extension (Phase II)
Pivotal Study (Phase III)
Solid tumors
Vaccine/Anti-muscle spasm/Anti-wrinkle
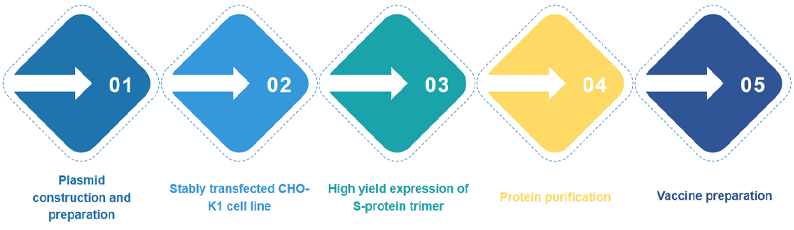
Unique protein purification process
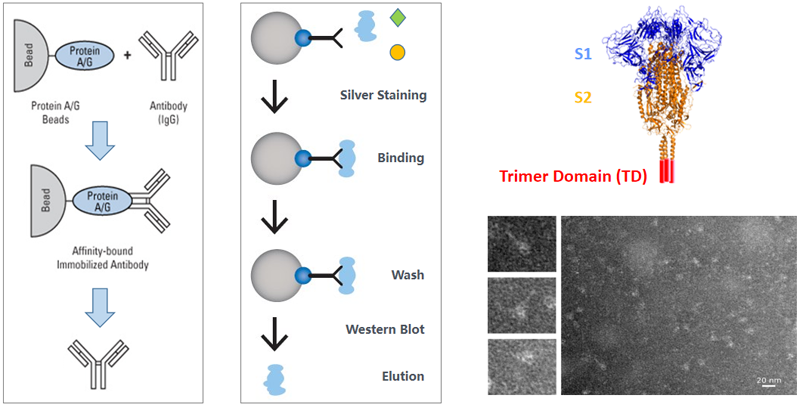
Protein Platform Progress
Candidates
Indication
Pre-clinical
IND
Dose Escalation (Phase I)
Dose Extension (Phase II)
Pivotal Study (Phase III)
/
Human and pets
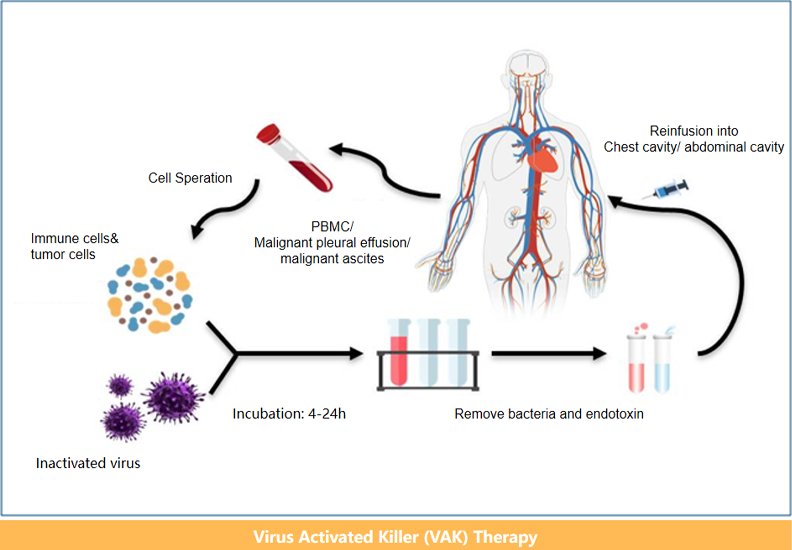
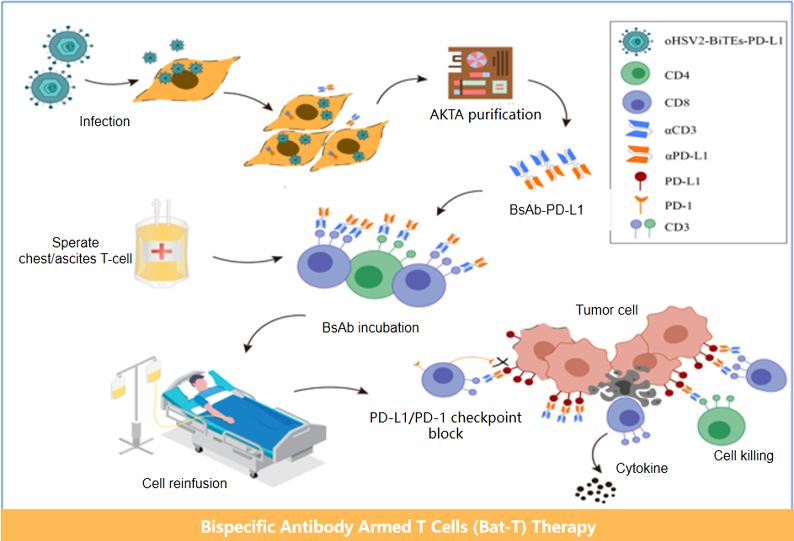



Cell Therapy Platform Progress
Candidates
Indication
Pre-clinical
IND
Dose Escalation (Phase I)
Dose Extension (Phase II)
Pivotal Study (Phase III)
Malignant pleural and abdominal effusions(IIT)
Malignant pleural and abdominal effusions