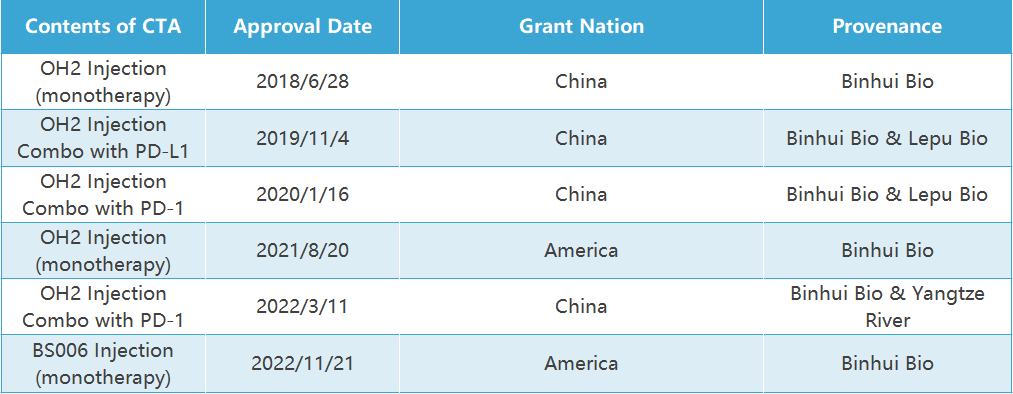
Successful IND application experience
Service
AdvantagesCore
advantagesSuccessful IND application experience
Products and services cover the whole life cycle of innovative drug R&D
Strongly supported by the four cross-coordinated platforms of viral vector, nucleic acid, protein, and cell therapy
Rich experience in process development and lab-scale/pilot scale production
8 GMP production lines, The production process conforms to FDA and NMPA standards
Overall quality management system with authentic, reliable, and traceable data
Comprehensive equipment, sufficient service space, and 60,000 m3 scalable and extensible R&D production base
Professional R&D, production and project management teams, and special personnel provides dedicated end-to-end services
Thorough implementation of the philosophy of Quality by Design (QbD)
Invention
patents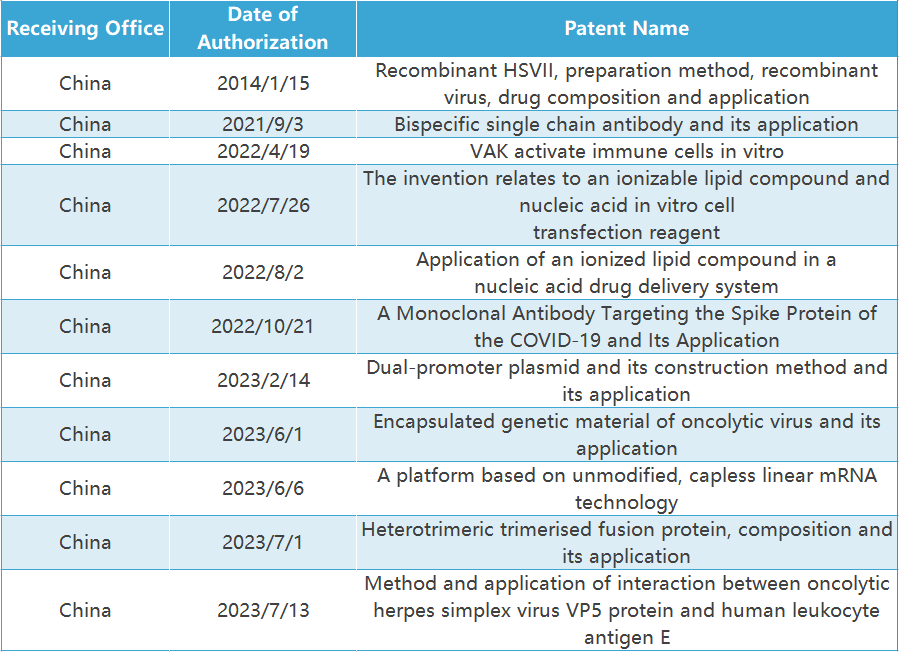
Clinical
approvals